Background
Chemotherapy refractoriness and disease relapse continue to be significant obstacles to therapeutic success in AML. We have recently identified bone marrow (BM) transcriptomic profiles that stratify patients with AML into an immune-infiltrated and an immune-depleted subtype and that refine the accuracy of survival prediction in response to conventional chemotherapy beyond that afforded by cytogenetic and molecular prognosticators (Vadakekolathu J, et al. Sci. Transl. Med. 2020; 12: eaaz0463). Importantly, AML CD8+ T cells exhibit features of immune exhaustion and senescence (IES), including the upregulation of natural killer (NK) cell-associated transcripts, which persist only in chemotherapy non-responders (Knaus HA, et al. JCI Insight 2018; 3: e120974). The aim of the current study was to determine whether IES correlate with immune infiltration and with clinical outcomes in AML.
Methods
We used prior knowledge and gene set enrichment analysis (GSEA) to derive a 68-gene IES signature score (computed as the cohort-wide average of gene expression) from publicly available RNA-sequencing datasets (TCGA and Beat-AML Master Trial; 162 and 281 AML patients, respectively). The wet-lab AML cohorts used in this study included a total of 382 BM samples from children and adults with AML treated with curative intent. BMs were collected at time of diagnosis, complete remission (CR) and relapse (PMCC, SAL and CHOP series). BM RNAs were profiled on the nCounter platform using the PanCancer Immune Profiling Panel (NanoString Technologies, Seattle, WA).
Results
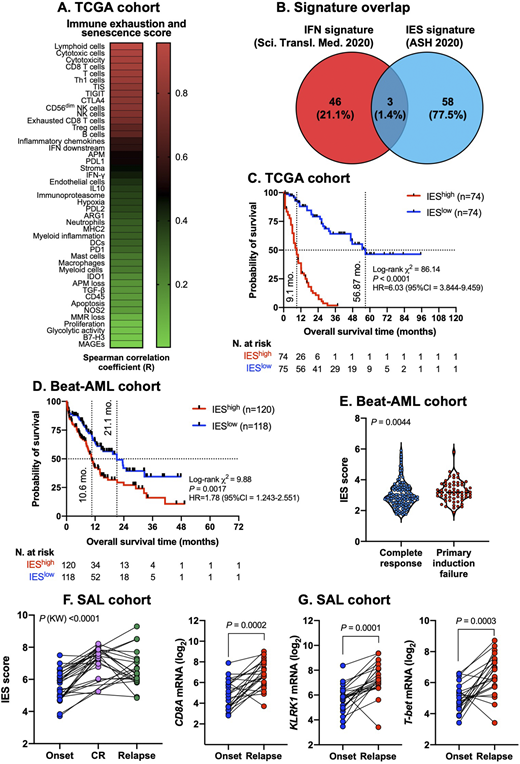
In the AML discovery cohort, the IES score significantly correlated with BM lymphoid infiltration (R=0.958) and with CD8+ T cell (R=0.88), cytotoxic T cell (R=0.90) and tumor inflammation signature scores (R=0.82, P<0.0001 for all; Fig. 1A). The novel IES gene signature showed minimal overlap with previously discovered interferon (IFN)-related gene sets with prognostic relevance in AML (Fig. 1B) and was higher in TP53-mutated (P<0.0001) and RUNX1-mutated (P=0.0071) compared with WT AML. Overall survival (OS) was significantly shorter in patients with higher than median IES scores (Fig. 1C). IES signature scores were also associated with worse clinical outcomes in Beat-AML cases (median OS of 10.6 and 21.1 months, respectively, in patients with higher and lower than median IES scores; Fig. 1D) and were higher in primary induction failures (PIF) compared with chemotherapy responders (Fig. 1E). The top IES genes associated with survival prediction both in TCGA and Beat-AML, although not overlapping with published IFN-related gene sets, were still significantly enriched in type I/II IFN signaling pathways and in molecular functions indicative of heightened ion-gated channel activity, as shown by protein-protein interaction network analyses. The above findings were validated in independent wet-lab cohorts comprising adults (PMCC series; n=290; SAL series; n=46) and children (CHOP series; n=46) with AML. In the ELN adverse risk subgroup (PMCC cohort), both relapse-free survival (RFS) and OS were significantly shorter in patients with higher than median compared with lower than median IES scores (median RFS time of 6.93 versus not reached [log-rank P=0.0053], and median OS of 10.5 months versus 18 months [log-rank P=0.0011], respectively). In contrast, the IES signature score failed to stratify survival in patients with ELN favorable and intermediate risk. Finally, a pairwise comparison of matched diagnostic, CR and relapse BM samples (SAL series; n=22 patients and CHOP series; n=46 patients) showed significantly higher IES signature scores at time of CR, congruent with chemotherapy-induced acceleration of IES, and at time of post-chemotherapy relapse compared with disease onset (Fig. 1F). Notably, CD8A, TBX21, a Th1 transcription factor, and markers of NK cells and cytotoxic T lymphocytes, including KLRK1, KLRD1, KLRC2, GNLY, and granzymes, were among the top ranked immune genes associated with AML relapse (Fig. 1G).
Conclusions
Patients with immune-infiltrated AML exhibit features of IES, which may correlate with adverse-risk molecular features, chemotherapy refractoriness and shorter survival. Molecular circuits reflective of IES might also underpin AML relapse after conventional induction chemotherapy. IES T cells could be functionally rejuvenated by novel immunotherapies being investigated in AML.
Church:NanoString Technologies, Inc.: Current Employment. Davidson-Moncada:Macrogenics: Current Employment. Tasian:Incyte Corporation: Research Funding; Aleta Biotherapeutics: Membership on an entity's Board of Directors or advisory committees; Gilead Sciences: Research Funding. Gojo:Amphivena: Research Funding; Merck: Research Funding; Genentech: Research Funding; Amgen: Research Funding; BMS: Membership on an entity's Board of Directors or advisory committees. Luznik:WindMil Therapeutics: Patents & Royalties: Patent holder; AbbVie: Consultancy; Merck: Research Funding, Speakers Bureau; Genentech: Research Funding. Rutella:Kura Oncology: Research Funding; MacroGenics, Inc.: Research Funding; NanoString Technologies, Inc.: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal